MicroRNAs in Autoimmune Sjögren’s Syndrome
Article information
Abstract
MicroRNAs (miRNAs), small non-coding RNAs, have been implicated in various diseases and cellular functions as microregulators of gene expression. Although the history of miRNA investigation in autoimmune Sjögren’s syndrome (SjS) is fairly short, a substantial amount of data has already been accumulated. These findings clearly indicate potential clinical implications of miRNAs, such as autoantigen expression and autoantibody production, viral miRNAs regulating the calcium signaling pathway, and aberrant immune cell regulation and cytokine production. Research endeavors in the field are currently underway to select disease-specific diagnostic and prognostic biomarkers by utilizing different types of tissues or biological specimens of SjS patients. Various techniques for miRNA analysis with different stringencies have been applied, with the most recent one being next-generation sequencing. This review compiles and highlights differentially-expressed miRNAs in various samples collected from SjS patients and their potential implications in the pathogenesis of SjS. To facilitate the development of miRNA-targeted personalized therapy in the future, we urge more follow-up studies that confirm these findings and elucidate the immunopathological roles of differentially-expressed miRNAs. Furthermore, improved diagnostic criteria for the disease itself will minimize sampling errors in patient recruitment, preventing the generation of inconsistent data.
Introduction
MicroRNAs (miRNAs) are single-stranded, non-coding small RNAs, whose sizes range from 18 to 25 nucleotides [1]. They play indispensable roles in regulating gene or protein expression by enhancing mRNA degradation or translational repression. It has been estimated that miRNAs control up to 60% of protein-coding genes in the human genome at the translational level [1]. Critical regulatory roles of miRNAs in complex cellular and physiological pathways have been extensively studied in various scientific fields. Examples of these roles include but are not limited to: differentiation, proliferation, migration, adhesion, apoptosis, hematopoiesis, and immune development and functional differentiation [2]. As they are involved in a variety of crucial regulatory functions, any abnormal expression and functions of miRNAs are predicted to have a profound impact on target molecules. For this reason, miRNA dysregulation has been implicated in a wide range of human diseases, such as cancer, neurodegenerative diseases, cardiovascular diseases, and autoimmune conditions. In this review, miRNAs in autoimmune diseases will be discussed with a special emphasis on autoimmune Sjögren’s syndrome (SjS). Translating the discovery of unique expression patterns and functions of miRNAs in SjS into clinical practice is expected to improve current diagnostic and therapeutic challenges.
Sjögren’s Syndrome
SjS is a chronic autoimmune disease that targets the exocrine glands of the salivary and lacrimal glands primarily, resulting in dry mouth and dry eye, respectively. Similar to many other autoimmune conditions, extraglandular manifestations are commonly seen in patients as well. Therefore, SjS patients may suffer from peripheral/autonomic neuropathy, cognitive impairment, gastroparesis, pancreatitis, autoimmune hepatitis, primary biliary cirrhosis, interstitial lung disease, vasculitis, arthritis, fibromyalgia, and Raynaud’s phenomenon [3–5]. Approximately, four million Americans are known to be affected by this condition. Current criteria for diagnosis mainly rely on the modified European and American diagnostic criteria proposed in 2002 [6], the SICCA criteria proposed in 2012 [7], and the American College of Rheumatology (ACR)/European League Against Rheumatism (EULAR) criteria proposed in 2016 [8]. In general, these guidelines consider serology for autoantibodies, ocular surface integrity evaluation, minor salivary gland (MSG) lip biopsy, and/or salivary and tear flow rates as main constituents of diagnosis.
Recent Studies on miRNAs in Autoimmune Diseases
With the growing evidence of the important biological roles of miRNAs in autoimmune diseases, such as immune cell lineage determination, differentiation, proliferation, and immune regulation, the identification of unique miRNA markers has provided invaluable information in understanding their potential roles in autoimmune disease pathogenesis. The complexity of the biological roles of miRNAs partly stems from the fact that a single miRNA targets multiple downstream molecules and a single target gene may be regulated by multiple miRNAs. In this section, recent studies on miRNAs in common autoimmune conditions and their potential roles are briefly described before extensive discussions on differentially expressed miRNAs in SjS.
Systemic sclerosis
In a recent miRNA study [9], serum samples of systemic sclerosis (SSc) patients (n = 26) were screened for 758 miRNAs, of which 30 miRNAs were identified as differentially upregulated miRNAs. Among these 30 miRNAs, miR-483-5p showed higher expression level in an independent SSc cohort, especially in early stages of SSc. The miRNA was also up-regulated in localized scleroderma, but not in systemic lupus erythematosus (SLE) or primary SjS (pSjS), indicating that this miRNA possibly contributes to the development of skin fibrosis. Furthermore, functional assays involving miR-483-5p overexpression in fibroblasts and endothelial cells revealed altered expression of fibrosis-related genes. This provides strong evidence that the serum miRNA miR-483-5p may function as a potential regulator of fibrosis in SSc.
Systemic lupus erythematosus
Peripheral blood mononuclear cell (PBMC) miRNA profiles in SLE (n = 8) were compared with those of pSjS (n = 8) and healthy controls (HC) (n = 7) by the Illumina next-generation sequencing technology (NGS) [10]. A total of 135 miRNAs were identified as differentially expressed in SLE compared to only 26 miRNAs in pSjS. Of 26 miRNAs differentially-expressed in pSjS, 25 miRNAs were also commonly overexpressed in SLE as well. Downregulation of miR-150-5p was only detected in pSjS, which appears to be associated with differential ratios of B cells in comparison with SLE [11]. Several miRNA molecules were overexpressed in SLE, but not changed in pSjS, which include miR-148a-3p, miR-152, miR-155, miR-223, miR-224, miR-326, and miR-342. Interestingly, expression levels of miR-223-5p, miR-150-5p, miR-155-5p and miR-342-3p were associated with the B cell subsets, such as native B cells or switched memory B cells in the PBMC samples. As B cell hyperactivity is characteristic in both pSjS and SLE, the miRNA expression signature may serve to dissect the pathological B cell regulation between those two conditions.
Rheumatoid arthritis
MiRNAs in synovial fluids and plasma in rheumatoid arthritis (RA, n = 40), osteoarthritis (OA, n = 38), and HC (n = 30) were analyzed by real-time quantitative reverse transcription-PCR (qRT-PCR) [12]. Synovial fluid concentrations of miR-16, miR-132, miR-146a, and miR-223 were significantly lower than their plasma concentrations, and there were no correlations between plasma and synovial fluid miRNAs. Interestingly, synovial tissues, fibroblast-like synoviocytes, and mononuclear cells secreted miRNAs in distinct patterns. Plasma miR-132 was significantly higher in HC than in RA or OA with high reliability, while synovial fluid concentrations of miR-16, miR-146a, miR-155, and miR-223 were significantly higher in RA than in OA. Plasma miRNAs or the ratio of synovial fluid miRNAs to plasma miRNAs, including miR-16 and miR-146a, significantly correlated with disease activity. Therefore, the authors proposed that synovial fluid and plasma miRNAs may serve as diagnostic biomarkers for RA and OA.
Early rheumatoid arthritis
Whole sera in early rheumatoid arthritis (ERA) patients (n = 34) were compared with established RA (n = 28) and HC (n = 16) in an analysis of miR-146a, miR-155, miR-223, miR-16, miR-203, miR-132 and miR-124a expression by TaqMan qRT-PCR [13]. The levels of circulating miR-146a, miR-155 and miR-16 were decreased in the sera of ERA patients in comparison with established RA. Levels of circulating miR-223 in treatment naive ERA patients correlated with disease activity. However, neither miR-16 nor miR-223 could distinguish ERA from HC. The authors concluded that circulating miR-146a, miR-155, and miR-16 in the sera of ERA could indicate an early stage of the disease.
Connective tissue diseases with interstitial lung disease
A recent study explored the correlation between miR-200c and the severity of interstitial lung disease (ILD) in patients (n = 218) with connective tissue diseases (CTDs) [14]. PBMCs were acquired from SSc (n = 23), dermatomyositis/polymyositis (DM/PM, n = 29), pSjS (n = 30), RA (n = 47), and HC (n = 23) for qRT-PCR analyses. The results indicated that the miR-200c level in the SSc group was significantly higher than in the DM/PM, pSjS, and RA groups. The level of miR-200c in the CTDs with ILD group was significantly higher than in the CTDs without ILD group, and the level in the severe ILD group was significantly higher than in the mild ILD group. The authors concluded that the level of miR-200c was positively correlated with the severity of ILD, proposing miR-200c in PBMCs as a biomarker of the severity of ILD in CTDs.
Differentially Expressed miRNAs in SjS
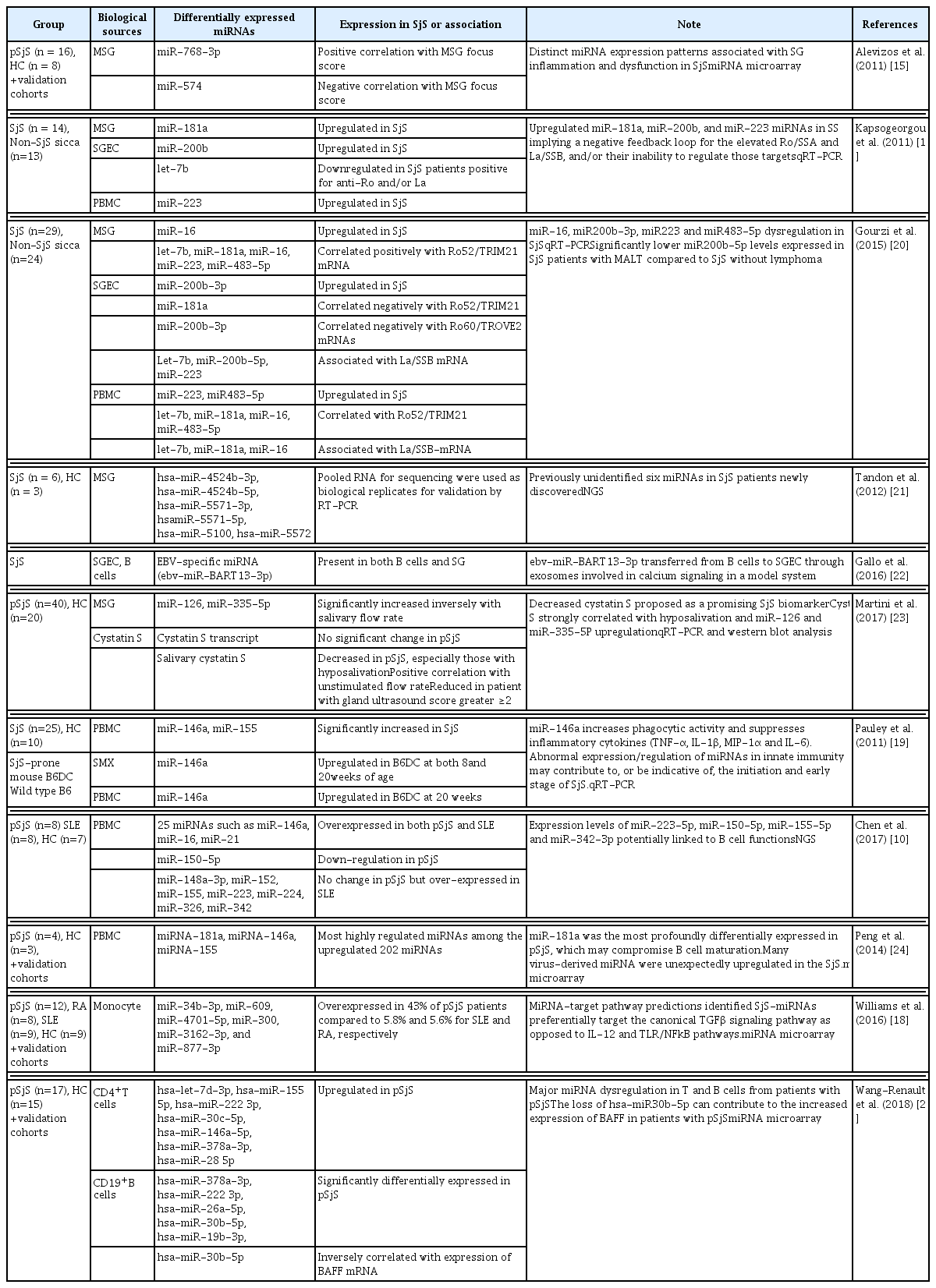
The history of investigating miRNAs in SjS is relatively short. The first journal article examining miRNAs in SjS was published only seven years ago in 2011 [15, 16]. Studies of miRNAs in SjS have utilized different types of samples, such as MSG collected from lip biopsies, sera, PBMC, or monocytes from blood ppub, and saliva or exosome from saliva ppub [15–19]. Utilization of mixed populations of cells or saliva that contains not only host-derived but also microorganism-derived miRNAs can cause difficulty in interpretation of the data. An interpretation of any published data on miRNAs will require some attention to the types of specimens and analysis methods utilized in a particular study. In addition, the exact implications of miRNAs in disease onset and regulation still await exploration. Nonetheless, the reported SjS-associated miRNA studies have provided valuable tools to discover the impact of miRNAs on SjS disease pathogenesis. This particular review is organized according to the category of specimens that have been utilized to investigate miRNA profiles in SjS. The intention of this approach was not necessarily to emphasize cell/target tissue specific regulation of miRNAs but to arrange the contents in a chronological manner, which happened to coincide with shifted interests in various sample utilization. Table 1 [10, 15, 16, 18–25] summarizes findings.
Minor salivary gland lip biopsy specimens
Microarray analyses have proven to be valuable in determining differentially-expressed miRNAs in the field. One of the first studies by Alevizos et al. [15] utilizing miRNA microarray for miRNAs in SjS MSG revealed a correlation between relative expression of selected miRNAs and the MSG focus score (FS). Among those miRNAs, miR-768-3p showed a positive correlation whereas miR-574 showed a negative correlation with FS, which was validated as markers of inflammation by qRT-PCR in an independent cohort. In addition, comparing miRNAs from patients with preserved vs. low salivary flow identified nine of differentially expressed miRNA, seven of which were up-regulated in the group with decreased salivary gland function. This suggests that the predicted targets of upregulated miRNA, which was associated with salivary dysfunction, may have a protective effect on epithelial cells.
Another comparative microarray analysis of miRNA expression in the MSGs of SjS and HC revealed distinctive miRNA signatures in SjS patients, associated with glandular inflammation and dysfunction [16]. MiRNAs that were predicted to target Ro/SSA and La/SSB autoantigens were upregulated in the MSG tissues (miR-181a), salivary gland epithelial cells (SGEC) (miR-200b) and PBMC (miR-223) of SjS in comparison with HC. miR-181a and miR-16 overexpression in MSG tissues of SjS patients has been previously shown to be associated with salivary dysfunction [15]. Interestingly, down-regulated expression of let-7b miRNA was observed in SGECs of SjS patients who were positive for Ro/SSA and/or La/SSB compared to seronegative patients. The authors hypothesized that the increased expression of miR-181a, miR-200b and miR-223 miRNAs in SjS may represent a negative feedback loop for the control of the elevated Ro/SSA and La/SSB expression and/or their inefficiency to regulate target expression.
In a subsequent study in 2015 by the same group, the authors further investigated 29 SjS patients (20 of 29 positive for anti-Ro/SSA and anti-La/SSB) and 24 non-SjS sicca controls to study possible associations between autoantigen mRNA expression and disease features [20]. The upregulated levels of miR-16 in MSGs, miR-200b-3p in SGECs, and miR-223 and miR-483-5p in PBMCs of SjS patients compared to non-SjS sicca controls were identified. Interestingly, the levels of miR-16 in MSGs and miR-483-5p in PBMC of SjS were not significantly altered in their previous study [16]. This may be due to the heterogeneous clinical features of SjS, resulting in subtle differences in the subjects recruited for those two studies. Nonetheless, the MSG and PBMC levels of let-7b, miR-16, and miR-181a were correlated positively with Ro52/TRIM21-mRNA. Significantly lower miR-200b-5p levels were also detected in SjS patients with mucosa-associated lymphoid tissue lymphoma compared to those SjS without lymphoma. The exact roles of dysregulated miRNAs in their study in promoting autoantigen expression and autoantibody production would be interesting to follow-up.
Recent high-throughput approaches such as NGS have been applied to sequence entire genomes or transcriptomes within days. This technology has yielded exciting discovery of unknown genes or small miRNAs with a challenge of mining large amounts of data. A study utilizing NGS revealed several previously unidentified miRNAs in the MSG of pSjS patients which were then validated in other cell lines [21]. The authors found hsa-miR-4524b-3p and hsa-miR-4524b-5p and hsa-miR-5100 particularly interesting to further pursue. Although the results were based on a relatively small number of patient samples, potentially due to cost-intensive NGS, from whole MSG biopsy specimens, thus including a mix of cells, the study showed a promising feasibility of discovering novel miRNAs by NGS.
A number of important contributions to the field of miRNA research have been made by identifying the functions of differentially-expressed miRNAs. An innovative study focusing on Epstein Barr virus (EBV) miRNA, ebv-miR-BART13-3p, indicated the presence of the miRNA in both B cells and SGEC in pSjS salivary glands [22]. As EBV typically infects B cells, the authors further demonstrated that ebv-miRBART13-3p can be transferred from B cells to SGEC through exosomes and that it targets stromal interacting molecule 1 (STIM1) and aquaporin 5 (AQP5), which are important regulators of salivary secretion via the Ca2+-mediated signaling pathway. The data suggest a mechanism by which EBV can contribute to secretory dysfunction in SjS. This might also explain why the degree of immune cell infiltration and secretory dysfunction are not always correlated in SjS. Additionally, the role of EBV in SjS pathogenesis needs to be revisited since it has been underestimated due to inconsistency in the prevalence, localization, or expression levels of virus in patients [26–30].
A recent study by Martini et al. investigated miR-126 and miR-335-5p in MSG biopsies and their known target molecule, cystatin S [23]. The results indicated that salivary cystatin S was significantly decreased in pSjS (n=40) vs. HC (n = 20), especially in those with reduced secretory function. A positive correlation was observed between salivary cystatin S and unstimulated flow rate. Salivary cystatin S was also significantly reduced in patients with a submandibular gland ultrasonography score ≥2. Interestingly, the expression levels of miR-126 and miR-335-5P increased inversely with salivary flow rate. The authors proposed cystatin S as a promising biomarker for pSjS as it strongly correlated with glandular dysfunction. The cystatin S transcript did not change significantly, suggesting posttranscriptional regulation.
Peripheral blood mononuclear cells
We have shown that both miR-146a and miR-155 were up-regulated in response to the adaptive immune response in PBMC derived from SjS when testing humans and mice [19]. In this study, miR-146a expression in SjS patients as well as in the SjS-prone C57BL/6.NOD-Aec1Aec2 mouse model was investigated to elucidate its involvement in SjS pathogenesis. Expression of miR-146a was significantly increased in SjS patients (n = 25) compared with HC (n = 10), and was upregulated in the salivary glands of the SjS-prone mouse at both 8 weeks (prior to disease onset) and 20 weeks (full-blown disease) of age, and in the PBMC at 20 weeks of age. More importantly, functional analysis revealed the roles for miR-146a in increasing phagocytic activity and suppressing inflammatory cytokine production (tumor necrosis factor α [TNF-α], interleukin [IL]-1β, macrophage inflammatory protein [MIP]-1α, and IL-6), whereas cell migration, nitric oxide production, and expression of antigen-presenting/costimulatory molecules were not affected in human monocytic THP-1 cells. Our data suggest that abnormal expression/regulation of miRNAs in innate immunity may contribute to, or be indicative of, the initiation and early stage of SjS [19].
MiR-146a is known to be activated by nuclear factor κB (NF-κB), which controls Toll-like receptor (TLR)/IL-1 receptor signaling pathway through TNF-associated factor 6 (TRAF6), IL-1 receptor-associated kinase (IRAK1), the signal transducer and activator of transcription 1(STAT1), and IRF5 [31]. It is important to mention that the expression, the roles, and the target gene regulation of miRNA146a/b need to be interpreted in the context of a specific disease entity as its regulatory mechanisms appear to be more complex than initially anticipated. For instance, in the PBMC of RA patients, the overexpression of miR-146a was found without any change in its target genes [31]. However, in SLE, the expression of miR-146a was decreased with increased IRAK1 expression and unchanged TRAF6 [32]. In a study by Zilahi et al. [33], the authors have found elevated miR-146a/b in the PBMC of SjS with down-regulated IRAK1 and upregulated TRAF6, detected by RT-PCR. Because of these findings, Jacob et al. [34] hypothesized that TRAF6 could be specific for SjS while IRAK1 could be regarded as a risk gene for SLE. They previously reported a multiple defect (decrease) in the expression of various protein kinase C isoenzymes (included PKC) in the monocytes and T cells of SLE patients, but not existing in SjS [10]. Therefore, the authors proposed that TRAF6 contributes to the activation of the NF-κB pathway by the involvement of PKC, which exists normally in the disease, and TRAF6 gene could be a new biomarker of SjS [33].
Another study discovered changes in miRNA expression profiles in the PBMC of patients with SLE (n = 8), pSjS (n = 8), and HC (n = 7) by the Illumina NGS technology [10], which was mentioned earlier for SLE in this review. This study by Chen et al. [10] is interesting in the sense that miR-146a was overexpressed in SLE patient PBMC, which is inconsistent with the finding by Tang et al. [32] that showed downregulated miR-146a in the SLE PBMC. Chen et al. [10] hypothesized that this inconsistency may be due to a difference in the ethnic backgrounds of participants in the two studies; Chen et al.’s study [10] involved Europeans and Tang et al.’s study [32] involved Chinese. The authors also indicated various external factors as a potential reason for the discrepancy, such as dietary habits, exposure to different infectious agents, and other environmental elements, which may have influenced miRNAs expression level in the PBMC of the patients [35, 36].
In a recent study by Peng et al. [24], several miRNAs with a viral origin including miR-181a were shown to be upregulated in PBMC of patients with SjS. MicroRNA profiling by microarrays in Chinese patients with pSjS revealed elevated miRNA 181a in the PBMC [24]. MiRNA expression was initially profiled in female patients (4 pSjS and 3 HC), followed by a large cohort of patients (n = 33) and HC (n = 10). Among the 202 miRNAs that were upregulated and 180 miRNAs that were downregulated in the patients with pSjS, the authors confirmed that miR-181a was the most profoundly differentially expressed in pSjS in comparison with HC. The authors speculate that elevated miR-181a levels in the PBMC of these patients compromise the maturation of B cells, thus enabling them to recognize and attack autoantigens and resulting in disease phenotypes [24]. Interestingly, many virus-derived miRNA were unexpectedly upregulated in the SjS patients, suggesting that viral infection of PBMC may play an important role in SjS.
Although the sources and compositions of the cells were different, the detection of viral miRNAs in PBMC by Peng et al. [24] was in line with the study investigating the salivary glands by Gallo et al. [22]. The former showing the presence of EBV miRNAs and the upregulation of miRNA-181a in B cells, where EBV establishes cell tropism, provides a convincing scenario that viral miRNAs may drive the expression of host miRNAs in a cell-specific manner. The latter study, mentioned earlier, demonstrated that ebv-miR-BART13-3p was present in both B cells and salivary epithelial cells in pSjS salivary glands. The authors further demonstrated the transfer of ebv-miR-BART13-3p from B cells to salivary epithelial cells through exosomes, suggesting a possibility of its role in compromising calcium signaling by targeting STIM1 and consequent secretory dysfunction. The establishment of the interplay between viral miRNA and host miRNA will provide the exact etiological and pathological link between viral infection and SjS.
Monocytes
Monocytes represent a relatively homogenous population of important precursor cells to peripheral macrophages and dendritic cells [18]. Monocytes have innate immune functions including immunomodulation, cytokine production, and phagocytosis [37]. In SjS, monocytes secrete pro-inflammatory cytokines such as IL-6 and B cell-activating factor (BAFF) upon stimulation [38], express type I interferon (IFN)-regulated genes [39, 40] with reduced expression of NFκB inhibitor (IκBα) [41], and show decreased phagocytosis of apoptotic cells [42]. Thus, monocytes reflect the inflammatory state in SjS patients and mature monocytes are proposed to contribute to salivary gland inflammation in SjS [43].
In our study published in 2016, monocytes purified from pSjS (n = 18) blood samples were compared by miRNA microarray with those purified from other disease controls of RA (n = 10), SLE (n = 10), and HC (n = 10) [18]. To validate selected miRNAs from the microarray analysis, the microarray cohort and a new cohort of monocyte RNA samples from 9 HC, 12 SjS, 8 SLE, and 9 RA patients were evaluated by qRT-PCR. We demonstrated that miR-34b-3p, miR-4701-5p, miR-609, miR-300, miR-3162-3p, and miR-877-3p were differentially expressed in 43% of pSjS patients compared to 5.8% and 5.6% for SLE and RA, respectively. Interestingly, the canonical transforming growth factor β (TGF-β) signaling pathway showed the greatest coverage of predicted targets, averaging 40.5% ± 23.1% pathway coverage for the six SjS-associated miRNAs. This suggests that these miRNAs preferentially target the canonical transforming growth factor β (TGFβ) signaling pathway as opposed to pro-inflammatory IL-12 and Toll-like receptor/NFkB pathways in SjS pathogenesis [18].
As our study was limited to RNA analyses, relationships between miRNAs and their post-transcriptional inhibition of target genes at the protein level need to be addressed as soon as sufficient human monocytes are collected. Nevertheless, SMAD4 gene expression exhibited repression in a subpopulation of pSjS patients by our SjS-associated miRNAs [18]. The SMAD4 protein of the family of TGFβ signaling molecules acts as an essential common mediator for receptor-regulated SMADs to enter the nucleus. Interestingly, miR-300 and miR-609 showed the strongest co-association by regression analyses and target prediction analyses, which indicates that SMAD4 may be targeted by SjS-associated miRNAs in a cooperative manner. To our knowledge, this is the only study where SjS monocytes were analyzed for miRNA profiling.
T and B lymphocytes
Dysregulated miRNA expression in purified T and B lymphocytes from patients with pSjS was reported this year [25]. The authors purified CD4+ T cells and CD19+ B cells from PBMC of two independent cohorts. Cohorts of pSjS and HC for discovery (n = 17 and n = 15, respectively) and another cohort for replication (27 for T cells and 25 for B cells for pSjS and 12 HC, respectively) were included in the study. The Exiqon system containing 372 miRNAs was utilized. In T lymphocytes, the study showed upregulation of haslet-7d-3p, hsa-miR-155-5p, hsa-miR-222-3p, hsa-miR-30c-5p, hsa-miR-146a-5p, hsa-miR-378a-3p and hsa-miR-28–5 p in both the discovery and the replication cohorts. In B lymphocytes, hsa-miR-378a-3p, hsa-miR-222-3p, hsa-miR-26a-5p, hsa-miR-30b-5p, and hsa-miR-19b-3p were significantly differentially expressed. Interestingly, expression of BAFF mRNA was inversely correlated with the down-regulated expression of hsa-miR-30b-5p in B cells from SjS patients. Furthermore, transfection of THP-1 cells with an antagomir (miRNA inhibitor) for hsa-miR-30b-5p led to a strong increase in BAFF expression, providing strong evidence that the loss of hsa-miR30b-5p could contribute to the increased expression of BAFF in patients with pSjS [25].
Tear, saliva, and serum
Biomarkers
A review article on biomarkers in pSjS by Chen et al. [44] emphasizes the importance of bodily fluids such as saliva, tear, and serum as ideal sources for biomarker studies. According to the review, a number of putative saliva biomarkers have been identified by quantitative proteomics, and the combined studies such as salivary proteomics and miRNA screening may improve the sensitivity and specificity of biomarkers for disease. The biomarkers identified in bodily fluids for pSjS, which is summarized in the review by Chen et al. [44], include but are not limited to: profilin and anhydrase I in saliva [45], IL-4 and IL-5 in saliva [46], Cathepsin S in tear [47], Myxovirus-resistance protein A (MxA) in whole blood [48], Fms-like tyrosine kinase 3 ligand (Flt-3L) in serum [49], and CXCL13 in serum and saliva [50].
It is well accepted that IFN type I signature in PBMC and the salivary glands serves as a valuable pSjS biomarker [51]. MxA level in monocytes and in whole blood has been correlated with IFN score and pSjS disease activity [48]. The level of MxA was reportedly reduced after treatment with hydroxychloroquine, which also supports the potentially important role of MxA as a disease biomarker [48]. Utilization of the enzyme immunoassay in the cited study suggested MxA <100 μg/L as low, and MxA >100 μg/L as high [48].
Flt-3L in serum might be associated with lymphoma development in SjS [49]. An interesting study by Tobon et al. [49] reported that the levels of Flt-3L were associated with previously-identified risk factors for lymphoma development, such as presence of purpura, lymphocytopenia, lower levels of C4 and IgM, higher levels of beta2 -microglobulin, and a higher disease activity score. Also, pSjS patients who had a history of lymphoma showed higher levels of Flt-3L. Moreover, the Flt-3L levels were increased in the serum up to about 8 years before the diagnosis of lymphoma. This study has suggested an ideal cutoff value of Flt-3L (175 pg/ml) for an association with lymphoma in patients with pSjS (44% for sensitivity, 97.5% for specificity, and 97% for negative predictive value).
Exosome
Exosomes are small membrane-bound macrovesicles (30–100 nm in size) secreted from various types of cells [52]. They resemble flattened spheres under the electron microscope [53, 54]. Exosomes are known to have a number of biological functions, such as intracellular communication, signal transduction, transport of genetic materials, and modulation of immune responses [54]. They can be found in various bodily fluids such as plasma, serum, breast milk, saliva, and urine [55]. Recent studies have reported miRNAs for SjS biomarkers in these fluids. It has been controversial whether miRNAs detected in many of those body fluids are circulating freely or are concentrated in exosomes [56].
To compare miRNA expression in the exosome pellet and the exosome-depleted fraction of serum and saliva, Gallo et al. [17] measured miRNA expression by qRT-PCR. The study examined miRNAs previously reported to be freely circulating or miRNAs known to be exosome-specific. The concentration of miRNAs was consistently higher in the exosome-pellet compared to the exosome-depleted supernatant. Selected miRNAs that are known to be detectable in exosomes were undetectable in whole serum and the exosome-depleted supernatant. Therefore, the study concluded that miRNAs are primarily present in exosomes. However, the authors found their data inconsistent with two other previously published studies; one indicating that miRNAs were predominantly found in exosome-free cell culture media [57], and another showing that miRNAs were bound to Argonaut-2 protein in plasma and serum [58]. The authors speculated that the discrepancy could be, in part, due to minor differences in purifying exosomes or differences between plasma and serum. The study emphasizes the utilization of exosomal miRNA for early biomarker studies to reduce false-negative results when detecting low abundance miRNAs and the importance of optimized exosome purification process.
Conclusion and Future Directions
Since the initial publications in 2011, numerous studies have discovered unique sets of miRNAs that may play a critical role in SjS disease pathogenesis. Some miRNAs have been reported as novel biomarkers for SjS while other miRNAs are uniquely expressed in certain tissues or in biological samples of SjS in comparison with controls; however, the specificity and sensitivity of SjS-associated miRNAs along with their functional implications are yet to be confirmed by in-depth analyses. MiRNA studies tend to utilize a relatively small sample size, partly due to cost-intensive high-throughput approaches; therefore, results should be confirmed in an independent cohorts of patients, ideally in collaboration with other centers or institutes.
In addition, as SjS is a clinically heterogeneous disease, selection of eligible patients for a study can be challenging. Furthermore, different assay systems with different stringency tend to yield inconsistent data among studies as well. Ever evolving diagnostic criteria can certainly add one more layer of complexity when it comes to patient-oriented studies. To minimize those challenges and avoid unnecessary confusions over the collected data, it would be desirable to establish a consensus over diagnostic criteria and subject eligibility among investigators before initiating a new study.
Translation of current discoveries will allow the identification of biomarkers and development of miRNA-based therapeutics in the near future. miRNA therapeutics are known to provide specificity, efficacy, and markedly reduced toxicity compared to other treatment modalities [59–61]. A development of miRNA-targeted molecular therapy in autoimmune diseases, especially for SjS, would be an exciting treatment option in the near future. Further understanding of the regulatory network of miRNA expression will expedite the development of the miRNA-targeted, personalized therapy. With different forms of RNAs such as long non-coding RNAs actively being investigated recent years, discoveries of non-coding RNA species that have been reported to date in the field of SjS may only be the tip of the iceberg.
Acknowledgments
This work was supported by the NIH/NIDCR grants, DE019644 and DE025726 (S.C.) and T90DE21990 (M.M.).
Notes
Authors’ contribution:
Conceptualization: SC, MM, KEL, DHK, KH
Funding acquisition: SC, MM
Writing – original draft: SC, MM, KEL
Writing – review & editing: SC, MM, KEL, DHK, KH
Conflict of interest
No potential conflict of interest relevant to this article was reported.